Healthspan Part 2- Exercise Benefits in Promoting Cardiovascular Function
Promoting Healthspan and Healthy Aging Through Exercise: Enhancing Cardiovascular Function
This is the second blog in our series on promoting healthspan and healthy aging through exercise. Here, we’ll focus on how exercise can significantly improve cardiovascular function, which plays a critical role in overall health and longevity.
Exercise can significantly improve cardiovascular function
which plays a critical role in overall health and longevity.
The Link Between Cardiovascular Health and Exercise
Cardiovascular diseases (CVDs) are the leading cause of mortality worldwide, with a sedentary lifestyle being one of the primary risk factors. Cardiac output and VO2max are two essential indicators of cardiovascular fitness that typically decline with age, directly affecting healthspan.
Cardiac Output and VO2max
Cardiac output refers to the amount of oxygen-rich blood pumped by the heart’s left ventricle per minute. Exercise has the potential to increase cardiac output up to four times in a healthy individual, and up to six to eight times in elite athletes. Similarly, VO2max measures the maximum amount of oxygen utilized by the body during exercise, serving as a critical marker for aging-related diseases. Research has consistently shown that endurance exercise can boost VO2max, regardless of age, sex, or type of exercise. In fact, athletes who maintain regular endurance training can sustain elevated VO2max levels well into their 80s.
One mechanism through which exercise promotes cardiovascular fitness is via physiological hypertrophy – an increase in heart muscle mass and enhanced contractile function. This type of growth differs from pathological hypertrophy, which can lead to heart failure. Physiological hypertrophy occurs due to increased stress on the heart wall during exercise, activating pathways involving insulin and insulin-like growth factor 1 (IGF1). In addition to hypertrophy, exercise also induces changes in the heart’s contractile apparatus, metabolism, and mitochondrial function, among other adaptations.
Cardiac Output and VO2max
Cardiac output refers to the amount of oxygen-rich blood pumped by the heart’s left ventricle per minute.
Exercise as a Tool to Combat Cardiovascular Disease
Regular endurance exercise is known for its preventive and therapeutic effects on several cardiovascular conditions, including myocardial infarction, atherosclerosis, and hypertension. A wealth of evidence suggests that consistent endurance exercise can delay the age-related decline of cardiac output and VO2max, thereby promoting disease prevention and enhancing quality of life.
The concept of “exercise as medicine” has gained widespread recognition over the past decade, resulting in numerous clinical studies exploring exercise regimens tailored to tackle cardiovascular challenges. Current recommendations for patients with cardiovascular diseases include moderate to low-intensity exercise (below 60% of maximal heart rate) performed three to five times a week. Notably, research has shown that long-term endurance exercise can aid recovery from heart conditions without exacerbating cardiac injury.
The Role of Exercise in Preventing Heart Failure
Heart failure is one of the top causes of death worldwide, and exercise has proven to be the most effective intervention for both prevention and treatment. Studies show that regular moderate-intensity exercise (around 60% of VO2max) can improve cardiac outcomes and enhance quality of life in patients with chronic heart failure. Although the precise mechanisms behind these benefits are not fully understood, exercise may positively impact heart failure by boosting energy metabolism, reducing oxidative stress, and improving mitochondrial function.
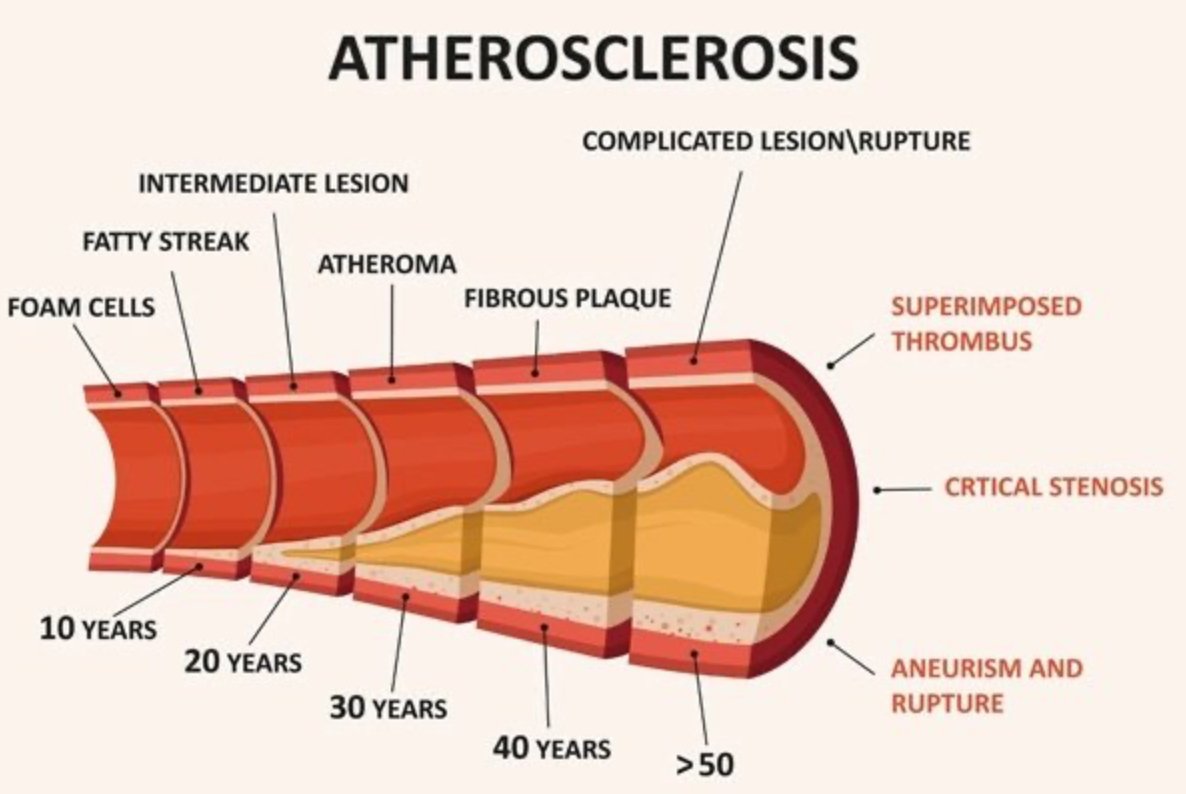
Atherosclerosis and Exercise
Atherosclerosis is a widespread vascular disease characterized by the accumulation of cholesterol-rich lipids in arterial walls, leading to inflammation and damage. Exercise has been found to improve endothelial function and exhibit anti-inflammatory and antioxidant effects, thus helping to prevent atherosclerosis.
Atherosclerosis
Exercise has been found to improve endothelial function and exhibit anti-inflammatory and antioxidant effects, thus helping to prevent atherosclerosis.
Exercise-Induced Stress Signaling and Cardiovascular Health
One key factor in the benefits of exercise on cardiovascular health is physiological stress signaling, primarily mediated by the 5’ AMP-activated kinase (AMPK) pathway. AMPK, activated during physical exertion, plays a crucial role in regulating cellular energy balance and cardiovascular health. Research suggests that specific subcellular localization of AMPK, particularly in mitochondria, might enhance the cardioprotective effects of exercise. Further studies are needed to explore these molecular pathways and their implications in exercise-induced cardiovascular benefits.
Mitochondria and Cardiovascular Health
Mitochondria, the powerhouses of the cell, play a central role in cardiovascular health. Exercise can improve both mitochondrial content (biogenesis) and function (dynamics such as fission, fusion, and mitophagy). The quality and efficiency of mitochondria are critical factors in protecting the heart from cardiovascular diseases. Regular exercise has been shown to enhance mitochondrial quality, measured by oxygen consumption, membrane integrity, and morphology, thereby contributing to cardiovascular health.
Muscle-Derived Factors in Exercise and Heart Protection
Endurance exercise also triggers the release of muscle-derived factors that can protect against heart disease. For example, extracellular superoxide dismutase (EcSOD), which is upregulated in skeletal muscles during exercise, can circulate to the heart and prevent conditions like diabetic cardiomyopathy.
The Takeaway: Exercise for Cardiovascular Health and Longevity
If your goal is to enhance your healthspan and longevity, incorporating cardiovascular exercise into your routine is essential. Physical activity offers numerous health benefits, regardless of age, sex, or ethnicity. It not only helps maintain a healthy weight but also supports everyday activities such as playing with your children or grandchildren, climbing stairs, and shopping, all of which are crucial for independent living.
In summary, the benefits of cardiovascular exercise extend far beyond general fitness. It can significantly reduce the risk of cardiovascular diseases, enhance heart function, and ultimately promote a longer, healthier life. The research is ongoing, but one thing is clear: regular physical activity is a cornerstone of healthspan and healthy aging.